Page 11 - reflections_newsletter10
P. 11
REFLECTIONS
Dyslipidaemia
Dyslipidaemia Global Newsletter #10 2025
Gene editing approaches, such as CRISPR-Cas9, base editing, and reverse transcriptase editing, enable direct modification or
inactivation of cholesterol-raising genes. In contrast, gene addition therapies, often delivered via adeno-associated viral (AAV)
vectors, insert functional copies of defective genes. Key molecular targets include PCSK9, ANGPTL3, APOC3, and LPA, which
Dyslipidaemia
play central roles in LDL-C, TG, and Lp(a) metabolism.
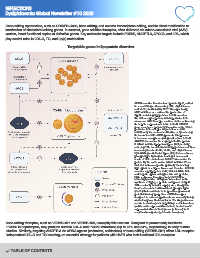
Targetable genes in lipoprotein disorders
APOB encodes the structural protein ApoB, critical
for assembling and secreting LDL, chylomicrons
and VLDL, facilitated by MTP. Therapeutically,
MTP inhibitors can reduce the production of
ApoB-containing lipoproteins. LDLR encodes
the LDL receptor protein, which promotes LDL
clearance, with therapies such as statins enhancing
its hepatic expression to lower LDL-C. PCSK9
gene encodes a protein by the same name, which
promotes LDL receptor degradation, and its
inhibition by monoclonal antibodies or by reducing
its translation with siRNA prevents this process,
to increase receptor recycling and reduce LDL-C.
ANGPTL3 encodes the angiopoietin-like protein
3, which inhibits lipoprotein lipase (LPL) activity,
reducing TGs breakdown from lipoproteins and thus
increasing levels of LDL, VLDL, and chylomicrons.
By inhibiting endothelial lipase (EL) it also reduces
phospholipids hydrolysis, leading to increased
levels of HDL cholesterol. ANGPTL4 encodes the
protein by the same name, which inhibits LPL as
well, but is tissue-specific, primarily modulating
lipid uptake in adipose tissue and muscles. APOC3
encodes apolipoprotein C-III, which inhibits LPL
and hepatic uptake of triglyceride-rich particles,
while antisense therapies target APOC3 to
lower triglycerides. LPA gene is involved in the
synthesis of Lp(a), a particle composed of ApoB-
100 covalently linked to apo(a) via a disulphide
bond and enriched with oxidised phospholipids
(OxPL) that contribute to its proatherogenic and
proinflammatory properties. siRNA therapies
designed to reduce Lp(a) levels are currently being
studied in phase 3 clinical trials. The diagram uses
arrows to show regulatory pathways and interactions
(solid arrows represent direct effect, dashed arrow
represents indirect effect) genes (in boxes) from
enzymes/transporter proteins (in hexagons).
Base-editing therapies, such as VERVE-101 and VERVE-102, exemplify this new era. Designed to permanently inactivate
PCSK9 in hepatocytes, they produce durable LDL-C and PCSK9 reductions (up to 73% and 84%, respectively) in early human
studies. Similarly, targeting ANGPTL3 via siRNA agents (zodasiran, solbinsiran) or base editing (VERVE-201) offers LDL receptor-
independent LDL-C and TG lowering, an essential strategy for patients with HoFH who lack functional LDL receptors.
TABLE OF CONTENTS